High-performance disintegrant for tablet formulation
Compared to conventional disintegrants such as SSG (sodium starch glycolate) and PVPP (polyvinylpolypyrrolidone), Disolcel® CCS offers superior disintegration and is effective even in small quantities of 0.5–3%. Thanks to its excellent water absorption capacity and rapid swelling properties, Disolcel® CCS is ideal for use in tablets, capsules, and granules.

Qualities of Disolcel® CCS
- Standard grade – General use
- GF grade (non-GMO) - In compliance with EC No. 1829/2003 and 1830/2003
For more information, please contact:

General benefits
- Rapid disintegration
- Aid in dissolution
- Ensuring the bioavailability of the active ingredient
- No impact on compressibility and flowability in the manufacturing process
- Cost-effective because of its low use level
Comparison of different disintegrants
In Mingtai’s research when usage level decreases to 0.25 % and 0.5 %, Disolcel® shows faster drug dissolution rate than other disintegrants. See graphics below.
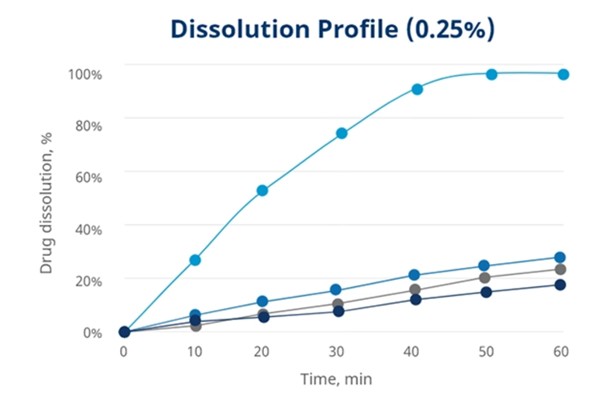
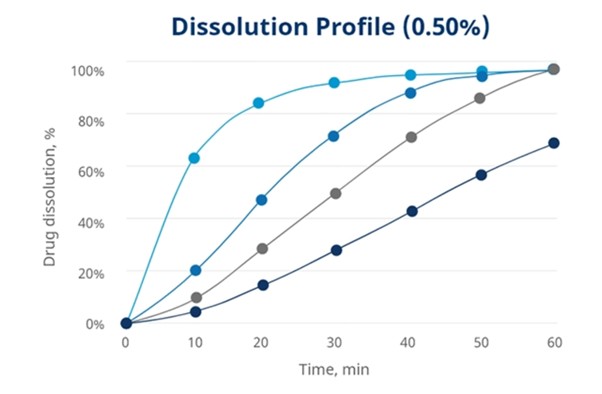

Benefits in formulation containing low solubility API
In order to facilitate a smooth production process, it is necessary to add some hydrophbic excipients to the formulations, such as glidant, lubricant and more. These hydrophobicexcipients usually have negative effects on tablet disintegration and drug dissolution.
The situation can be even worse for those API with low solubility.
With the increase of hydroophobic property in the formulation system, it becomes more difficult for the water to penetrate the tablet whick will cause disintegration time to increase dramatically.
Disolcel® CCS is less sensitive to hydrophobic property of the tablet system which makes it a better disintegrant solution.
In an experiment, the hydrophobic excipient talc is mixed in various ratios with other hydrophilic excipients to create systems with different hydrophobic properties.
The disintegration time of tablets containing 1% Disolcel® CCS in different talc ratios is similar.
The situation remains unchanged for day 0 and day 7 when stored under conditions of 40°C/75% RH.
In contrast, the disintegration time of tablets containing 1% PVPP increases with increasing hydrophobic properties in the system.
The disintegration time for day 0 and day 7 (40 °C/75% RH) also increases with the addition of PVPP. See graphs below.
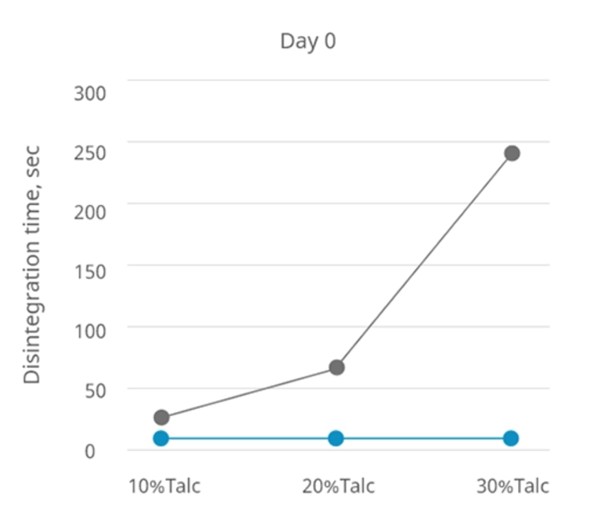
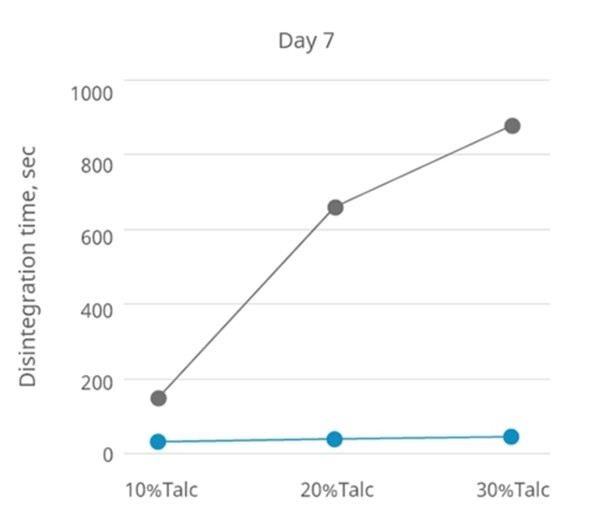
Conclusion
Thanks to its superior properties, Disolcel® CCS is the ideal choice for formulations requiring robust tablet strength, stable production processes, and reliable disintegration times under demanding conditions.
Distributed in:
Austria, Croatia, Czech Republic, Denmark, Estonia, Finland, Germany, Ireland, Latvia, Lithuania, Macedonia, Norway, Poland, Serbia, Slovakia, Slovenia, Sweden and Switzerland.


High-performance disintegrant for tablet formulation

Gelest Appoints Nordmann as Primary EMEA Distribution Partner



